Background
Australian marsupials face high extinction rates due to climate change, fires, non-native predators, habitat degradation, and diseases (Fisher et al., 2014; Lindenmayer & Dickman, 2022). Despite extensive research and management, innovative, long-term genetic preservation strategies are vital (Legge et al., 2023). To address this challenge, it is crucial to develop and integrate ground-breaking approaches involving genetic management with modern assisted reproductive methods like in vitro embryo production and the preservation of gametes, embryos, and somatic cells. (Andrabi & Maxwell, 2007)
Viable cellular material from deceased marsupials can be obtained through different methods such as epididymal mincing in males for collecting sperm (Ali Hassan et al., 2021), harvesting oocytes from female ovaries (Huijsmans et al., 2023) and isolating somatic cells from skin tissue (Korody et al., 2021) that can be potentially differentiated into stem cells.
The potential to preserve cells or gametes at -196ºC or room temperature enables the creation of genome resource banks and facilitates genetic transport (Bolton et al., 2022; Keskintepe & Eroglu, 2021). Preserved sperm, oocytes, and cells can later be used for laboratory-based embryo production by techniques such as in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), somatic cell nuclear transfer (SCNT) and recent advancements enabling the use of skin cells for producing embryos, particularly for endangered species (Korody et al., 2021). There is currently limited knowledge on collecting and preserving marsupial gametes and embryos in a lab setting. Progress in these technologies not only safeguards their genetics but also enhances our understanding of marsupial reproductive physiology.
Aims
- To develop post-mortem protocols for collecting, assessing, and preserving sperm, oocytes, and somatic cells from deceased marsupials.
- To refine in-vitro embryo production techniques for diverse marsupial species, using the Kangaroo as our main animal model.
- To establish in vitro-somatic cell growth conditions for different marsupials.
- To innovate marsupial genetic preservation via freeze-drying for sperm and somatic cells.
Methodology
For deceased males, the epididymis (where the sperm is normally accumulated) is carefully separated from the testicle and minced with a scapple into small pieces (Figure 1A) and kept on a Petri dish with media. After some incubation (Figure 1B), a comprehensive sperm assessment is conducted (Figure 1D), and the subsequent steps involve either sperm cryopreservation or lyophilization for preservation (Figure 1E).
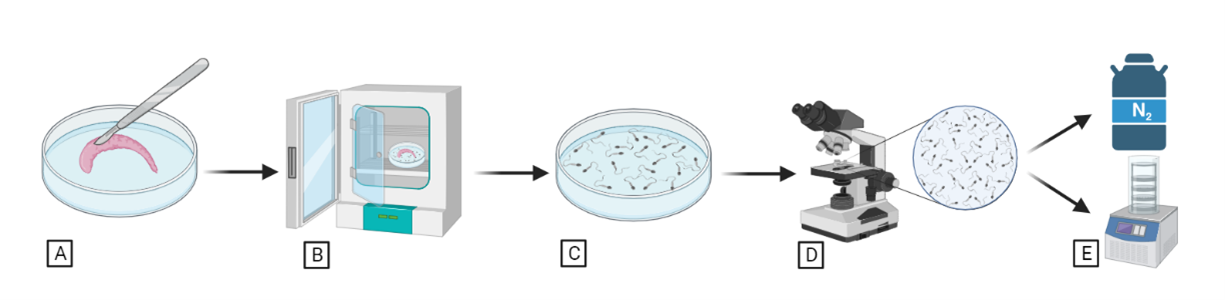
Figure 1. Sperm collection, assessment, and preservation from epididymis of deceased males.
For a deceased female, oocytes will be retrieved from the ovaries through a process involving needle punctures and microscopic incisions across the entire ovarian surface to allow oocytes to be released from the follicles (Figure 2A, Figure 2B). Subsequently, oocytes are searched under the stereoscope, and their morphology is assessed to evaluate oocyte quality (Figure 2C). Oocytes are either fixed for further studies or matured in vitro for future in vitro embryo production (Figure 2D)
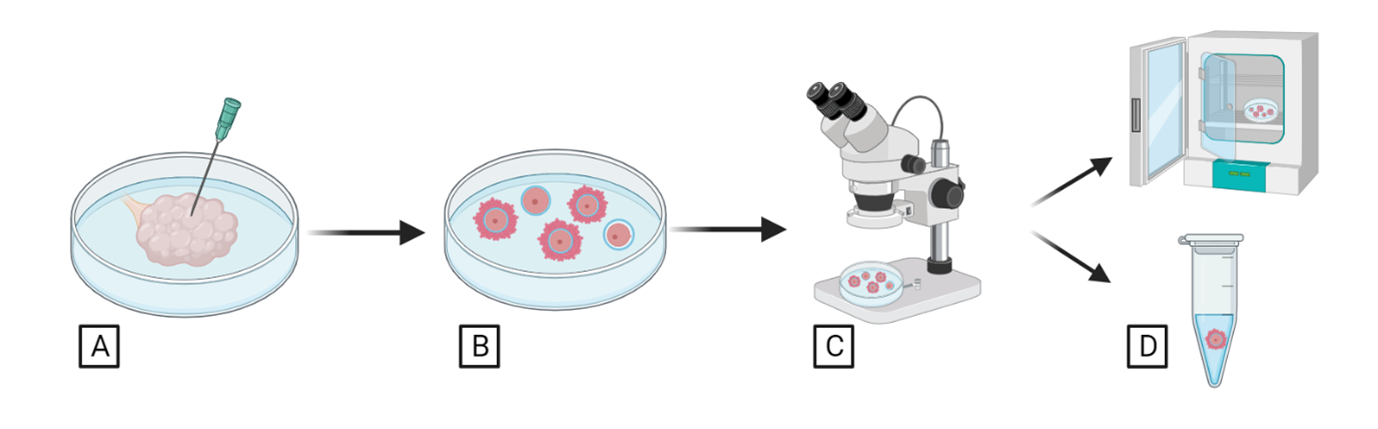
Figure 2. Oocyte collection and assessment from ovaries of deceased females.
Somatic cell culture entails the retrieval of small skin samples for future incubation to promote fibroblast growth at varying temperatures (Figure 3A, Figure 3B). Cellular progress will be assessed at 48-hour intervals (Figure 3C, Figure 3D). Upon achieving sufficient growth, cells will be detached and prepared for cryopreservation or lyophilization for preservation (Figure 3E).
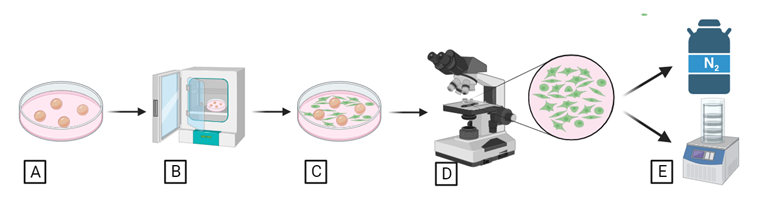
Figure 3. Somatic cell culture and preservation.
Expected Outcomes
By pioneering techniques for sperm preservation, oocyte maturation, and ICSI in a marsupial model, we will enhance our foundational knowledge of marsupial gamete biology and early embryo development. The current proposal will translate these new developments to marsupials for the first time. The project represents a revolutionary shift in the approach to marsupial conservation biology and establishes the essential technologies necessary for first functional genome resource bank for the taxa. Not only will this research provide fundamental insight to marsupial gamete and early embryo development biology, but it will also advance its application for conservation of endangered Australian wildlife.
References
Ali Hassan, H., Domain, G., Luvoni, G. C., Chaaya, R., Van Soom, A., & Wydooghe, E. (2021). Canine and feline epididymal semen—a plentiful source of gametes. Animals, 11(10), 2961.
Andrabi, S. M. H., & Maxwell, W. M. C. (2007). A review on reproductive biotechnologies for conservation of endangered mammalian species. Animal Reproduction Science, 99(3), 223-243. https://doi.org/https://doi.org/10.1016/j.anireprosci.2006.07.002
Bolton, R. L., Mooney, A., Pettit, M. T., Bolton, A. E., Morgan, L., Drake, G. J., Appeltant, R., Walker, S. L., Gillis, J. D., & Hvilsom, C. (2022). Resurrecting biodiversity: advanced assisted reproductive technologies and biobanking. Reproduction and Fertility, 3(3), R121-R146.
Fisher, D. O., Johnson, C. N., Lawes, M. J., Fritz, S. A., McCallum, H., Blomberg, S. P., VanDerWal, J., Abbott, B., Frank, A., & Legge, S. (2014). The current decline of tropical marsupials in A ustralia: is history repeating? Global Ecology and Biogeography, 23(2), 181-190.
Huijsmans, T. E., Hassan, H. A., Smits, K., & Van Soom, A. (2023). Postmortem Collection of Gametes for the Conservation of Endangered Mammals: A Review of the Current State-of-the-Art. Animals, 13(8), 1360.
Keskintepe, L., & Eroglu, A. (2021). Preservation of Mammalian Sperm by Freeze-Drying. Methods Mol Biol, 2180, 721-730. https://doi.org/10.1007/978-1-0716-0783-1_39
Korody, M. L., Ford, S. M., Nguyen, T. D., Pivaroff, C. G., Valiente-Alandi, I., Peterson, S. E., Ryder, O. A., & Loring, J. F. (2021). Rewinding extinction in the northern white rhinoceros: genetically diverse induced pluripotent stem cell bank for genetic rescue. Stem Cells and Development, 30(4), 177-189.
Legge, S., Hayward, M., & Weeks, A. (2023). Novel conservation strategies to conserve Australian marsupials. In American and Australasian Marsupials: An Evolutionary, Biogeographical, and Ecological Approach (pp. 1-30). Springer.
Lindenmayer, D., & Dickman, C. (2022). Impact of Habitat Loss and Fragmentation on Assemblages, Populations, and Individuals of Australasian Marsupials. In American and Australasian Marsupials: An Evolutionary, Biogeographical, and Ecological Approach (pp. 1-32). Springer.
Acknowledgements
Hidden Vale Conservation Research Support grant 2023 and 2024
Hidden Vale Staff members
Schemes were produced using Bio Render





